Existing first-line treatments have declining utility in combating UTI, highlighting the need for innovative alternatives to better serve patients
Effective first-line options for UTI treatment are becoming more and more limited1
For decades, antibiotics have been a mainstay of UTI treatment, but the growing threat of antibiotic resistance has limited the options clinicians have available to effectively control infections.2,3 If not managed effectively, UTIs can lead to greater illness and prolonged discomfort for patients as well as a high rate of treatment failure, requiring additional rounds of treatment and/or hospitalization.4
These therapeutic challenges negatively impact patient outcomes, add to the economic burden of treatment, and contribute further to health disparities experienced by women in the United States.4,5
UTIs are becoming increasingly resistant to multiple antibiotics
Antibiotic resistance is one of the greatest threats to health in the United States and globally.6 New resistant strains of common bacteria are emerging, making traditional antibiotics less effective at treating common infections.6
Infection with antibiotic resistance can lead to serious illnesses such as progression to sepsis or septic shock in the case of UTI, prolonged hospital admissions, treatment failure, and death.7 Over a period of 13 years, as the rate of resistance continued to grow, the number of UTIs requiring hospitalization increased by 76%, dramatically driving up treatment costs.1
In the United States, almost 3 million antibiotic-resistant infections occur each year.8
Antibiotic resistance remains one of the most significant health challenges. There is a great need for new antibiotic therapies.
40% of women in the United States will develop a UTI at some point in their lifetime.10
Women are disproportionately impacted by UTIs, making up 99% of those affected by uncomplicated UTIs9
The symptoms of UTI can have a significantly detrimental impact on women’s quality of life. The painful and uncomfortable symptoms often cause physical and emotional distress as well as disruption to daily activities, social interactions, and work.4
Given the health inequalities currently faced by millions of women, it’s imperative to limit any additional disparities in care.
UTILITY therapeutics has acquired the US rights to 2 European-approved antibiotics offering a new type of treatment with a unique mode of action — novel in the US market — to patients suffering from UTIs.
Enterobacteriaceae is a gram-negative family of bacteria, including E coli, that are a common cause of numerous infections in humans.11,12 In fact, most UTIs are caused by E coli.12 Over the past decade, a growing number of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, a group of enzymes that render some antibiotics ineffective, have been emerging and spreading throughout the world.11,12
Pivmecillinam (oral) and mecillinam (IV) are a unique class of antibiotics with potent in vitro and in vivo activity against the most common bacteria in UTI, including resistant strains such as ESBL-producing E coli.
For more than 40 years, pivmecillinam (oral) and mecillinam (IV) have been used outside of the United States to successfully treat UTIs and are an integral part of the patient care pathway globally.13,14 The treatment is recommended for first-line use in many countries and is part of the Infectious Diseases Society of America (IDSA) and the European Society for Microbiology and Infectious Diseases guidelines.13,15,16
The novel mode of action of pivmecillinam (oral) and mecillinam (IV) supports decreased resistance
Pivmecillinam (oral) is an aminopenicillin, which is a specific class of beta-lactam antibiotics with a distinct mechanism of action.17 Pivmecillinam is the prodrug of mecillinam and is rapidly converted into mecillinam in vivo. Due to its unique MOA, PIVYA™ (pivmecillinam, oral) may address the resistance issues facing other treatments in the United States.
Clinically, the reason for the low resistance, as recently uncovered in peer-reviewed research, is the collateral sensitivity of pivmecillinam (oral), which is an evolutionary trade-off between pivmecillinam (oral) resistance and resistance towards other beta-lactam antibiotics.18 As a result, PIVYA™ (pivmecillinam, oral) can provide further benefits to the fight against rising levels of antibiotic resistance.
Real-world experience adds to the wealth of existing evidence. In Denmark, pivmecillinam (oral) has been a mainstay treatment of UTIs since the 1970s and is the most frequently used systemic antibiotic in the country.14,19 While the treatment is being used for 80% of all UTIs, the resistance levels have remained in the low single digits.20
Mecillinam (IV) solely targets penicillin binding protein-2 (PBP-2) in the cell wall of gram-negative bacteria.17,21 This unique mechanism leads to favorable stability against β-lactamase hydrolysis compared to other penicillins.17,21
Development program for PIVYA™ (pivmecillinam, oral)
UTILITY therapeutics is working with the Food and Drug Administration to make PIVYA™ (pivmecillinam, oral) tablets available for the treatment of uncomplicated UTIs. Although not yet approved for use in the United States, pivmecillinam (oral) has been recommended as a first-line treatment of uncomplicated UTI by the Infectious Diseases Society of America (IDSA)15 based on the depth and breadth of existing evidence and clinical experience outside of the United States. Encouraged by this positive adoption by the IDSA, UTILITY therapeutics is excited to bring this potential new first-line treatment to patients and clinicians in the United States.
A development program for mecillinam (IV) in the treatment of complicated UTIs is also underway.
These endeavors represent our unwavering commitment to advancing medical solutions and enhancing the lives of those in need.
Product Pipeline
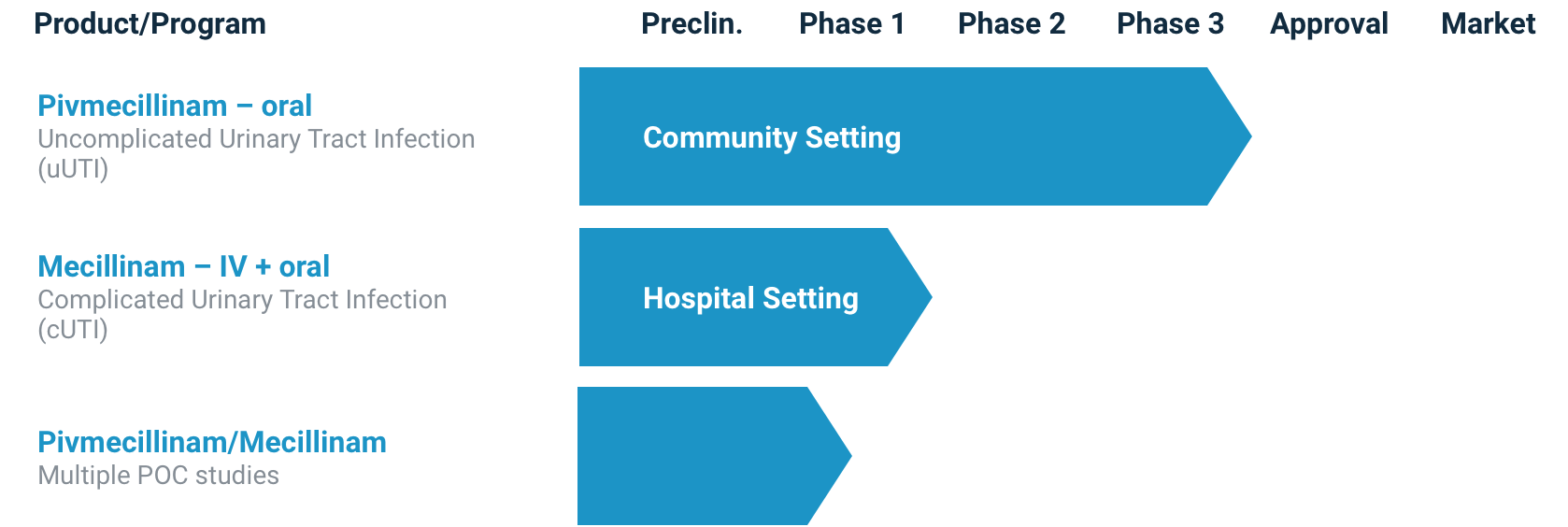
Changing the way UTIs are treated in the United States
Based on strong evidence showing consistent activity against key resistant pathogens, well-established clinical data, and extensive real-world experience from Europe in treating UTIs, PIVYA™ (pivmecillinam, oral) and mecillinam (IV) have the potential to become first-line treatment options of choice, offering new therapies to patients across the United States.
PIVYA™ (pivmecillinam, oral) has the potential to be a first of its kind, innovative, first-line antibiotic in the United States for the treatment of uncomplicated UTI
Treating uncomplicated UTI remains a significant hurdle for clinicians, as there are limited options for viable first-line therapies that are effective, safe, and do not contribute to antimicrobial resistance.
In clinical studies* pivmecillinam (oral) has demonstrated a strong safety and efficacy profile. In its more than 4 decades of real-world usage in Europe, clinical cure rates with pivmecillinam (oral) have been shown to be up to 95%23-26 with no serious adverse events observed in more than 30 million courses administered.27
PIVYA™ (pivmecillinam, oral) may also offer clinical benefit in the treatment of complicated UTI as an IV to oral step-down therapy to treat complicated UTI in hospitals and enable more rapid discharge of patients.
In recognition of the unmet medical need in UTI, the FDA has granted PIVYA™ (pivmecillinam, oral) status as a Qualified Infectious Disease Product (QIDP),27 which provides expedited regulatory review and an additional 5 years of market exclusivity.
*The New Drug Application submitted to the US FDA for PIVYA™ (pivmecillinam, oral) comprises 6 clinical studies supporting the efficacy and 12 clinical studies supporting the safety of PIVYA. Through a sequence of interactions with the FDA, UTILITY has reanalyzed all available data according to the FDA 2019 uncomplicated UTI guidance. The clinical data support first-line positioning due to its benign safety profile and consistent efficacy.
Mecillinam (IV) has been a fundamental and essential part of infection treatment globally, playing a crucial role in managing and treating infections, primarily UTI
Unlike uncomplicated UTIs, complicated UTIs can be difficult to treat, as there are generally other comorbidities that warrant more urgent intervention, and antibiotic resistance remains a critical impediment to care.28
Mecillinam (IV) has been recognized as a significant and important antimicrobial drug for over 40 years.14 Used to treat infections caused by susceptible gram-negative bacteria, mecillinam (IV) has demonstrated consistently low and stable resistance (~5%).29 Furthermore, the likelihood of developing cross-resistance to other beta-lactam antibiotics is minimal.29 The available clinical evidence suggests that, since its inception, global resistance to mecillinam (IV) among bacteria responsible for UTIs has remained extremely low.29
If approved, mecillinam (IV) could potentially be a useful tool for healthcare professionals in the United States.
Learn about the latest
developments
References: 1. Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998-2011. Open Forum Infect Dis. 2017;4(1):ofw281. doi:10.1093/ofid/ofw281 2. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269-284. doi:10.1038/nrmicro3432 3. Salam MA, Al-Amin MY, Salam MT, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare (Basel). 2023;11(13):1946. doi:10.3390/healthcare11131946 4. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol. 2019;11:1756287219832172. doi:10.1177/1756287219832172 5. Alcalde-Rubio L, Hernández-Aguado I, Parker LA, Bueno-Vergara E, Chilet-Rosell E. Gender disparities in clinical practice: are there any solutions? Scoping review of interventions to overcome or reduce gender bias in clinical practice. Int J Equity Health. 2020;19(1):166. doi:10.1186/s12939-020-01283-4 6. Antibiotic resistance. World Health Organization. July 2020. Accessed October 17, 2023. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance 7. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903-3910. doi:10.2147/IDR.S234610 8. CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: Centers for Disease Control and Prevention; 2019. doi:10.15620/cdc:82532 9. American Urological Association. Medical Student Curriculum: Adult UTI. April 2020. https://www.auanet.org/meetings-and-education/for-medical-students 10. Bono MJ, Leslie SW, Reygaert WC. Urinary tract infection. StatPearls Publishing; November 28, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470195/ 11. Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One. 2019;14(12):e0220265. doi:10.1371/journal.pone.0220265 12. Kumar D, Singh AK, Ali MR, Chander Y. Antimicrobial susceptibility profile of extended spectrum ß-lactamase (ESBL) producing Escherichia coli from various clinical samples. Infect Dis (Auckl). 2014;7:1-8. doi:10.4137/IDRT.S13820 13. Lodise TP, Henriksen AS, Hadley T, Patel N. US-focused conceptual health care decision-analytic models examining the value of pivmecillinam relative to current standard-of-care agents among adult patients with uncomplicated urinary tract infections due to Enterobacterales. Open Forum Infect Dis. 2021;8(10):ofab380. doi:10.1093/ofid/ofab380 14. Firmodt-Moller N. Mecillinam – reversion of resistance and how to test it. EBioMedicine. 2017;23:4-5. doi:10.1016/j.ebiom.20177.08.023 15. Gupta K, Hooton TM, Naber KG, et al; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-20. doi:10.1093/cid/ciq257 16. European Association of Urology. EAU guidelines on urological infections. 2023. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urological-infections-2023.pdf 17. Kresken M, Pfeifer Y, Wagenlehner F, Werner G, Wohlfarth E; ‘Antimicrobial Resistance’ of the Paul Ehrlich Society for Infection Therapy. Resistance to mecillinam and nine other antibiotics for oral use in Escherichia coli isolated from urine specimens of primary care patients in Germany, 2019/20. Antibiotics (Basel). 2022;11(6):751. doi:10.3390/antibiotics11060751 18. Rosenkilde CEH, Munck C, Porse A, Linkevicius M, Andersson DI, Sommer MOA. Collateral sensitivity constrains resistance evolution of the CTX-M-15 β-lactamase. Nat Commun. 2019;10(1):618. doi:10.1038/s41467-019-08529-y 19. Holm A, Cordoba G, Aabenhus R. Prescription of antibiotics for urinary tract infection in general practice in Denmark. Scand J Prim Health Care. 2019;37(1):83-89. doi:10.1080/02813432.2019.1569425 20. Korsgaard HB, Ellis-Iversen J, Hendriksen RS, et al. DANMAP 2019 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2020. ISSN 1600-2032. 21. Dewar S, Reed LC, Koerner RJ. Emerging clinical role of pivmecillinam in the treatment of urinary tract infection in the context of multidrug-resistant bacteria. J Antimicrob Chemother. 2014;69(2):303-308. doi:10.1093/jac/dkt368 22. Bjerrum L, Gahrn-Hansen B, Grinsted P. Pivmecillinam versus sulfamethizole for short-term treatment of uncomplicated acute cystitis in general practice: a randomized controlled trial. Scand J Prim Health Care. 2009;27(1):6-11. doi:10.1080/02813430802535312 23. Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: the LUTIW project. Scand J Prim Health Care. 2007;25(1):49-57. doi:10.1080/02813430601183074 24. Menday AP. Comparison of pivmecillinam and cephalexin in acute uncomplicated urinary tract infection. Int J Antimicrob Agents. 2000;13(3):183-187. doi:10.1016/s0924-8579(99)00118-1 25. Nicolle LE, Madsen KS, Debeeck GO, et al. Three days of pivmecillinam or norfloxacin for treatment of acute uncomplicated urinary infection in women. Scand J Infect Dis. 2002;34(7):487-492. doi:10.1080/00365540110080728 26. Vik I, Bollestad M, Grude N, et al. Ibuprofen versus pivmecillinam for uncomplicated urinary tract infection in women—a double-blind, randomized non-inferiority trial. PLoS Med. 2018;15(5):e1002569. doi:10.1371/journal.pmed.1002569 27. Data on File. UTILITY therapeutics. 28. Marantidis J, Sussman RD. Unmet needs in complicated urinary tract infections: challenges, recommendations, and emerging treatment pathways. Infect Drug Resist. 2023;16:1391-1405. doi:10.2147/IDR.S382617 29. Al-Sarraj FM, Al-Hejin AM, Jiman-Fatani A, Qari MH. Mecillinam and fosfomycin susceptibility in multi-drug-resistant Escherichia coli isolated from urine samples. Br J Med Health Res. 2019;6(07). doi:10.46624/bjmhr.2019.v6.i07.007